The Newest Pillar of Cancer Care
Cancer care has long been dominated by three pillars of treatment: surgery, chemotherapy and radiation. In recent years, oncologists have added another one: cancer immunotherapy, which harnesses the power of the patient’s own immune system to detect and destroy cancer.
Cellular therapy is a form of cancer immunotherapy that involves genetically engineering immune cells to recognize, target and destroy only cancerous cells, leaving healthy cells intact. In contrast, chemotherapy and radiation attack both cancerous and healthy cells, causing debilitating side effects. In addition, some cancers are resistant to chemotherapy and radiation. Cellular therapy offers another option for some patients.
There are several types of cellular therapy used to treat cancer, including one approved for pediatric use: chimeric antigen receptor T cell therapy, known as CAR-T cell therapy.
Renewed hope for children with cancer
In 2017, CAR-T cell therapy received FDA approval for use in children with relapsed or treatment-resistant B-cell acute lymphoblastic leukemia (ALL). Approximately 85% to 90% of ALL patients are treated successfully with standard chemotherapy. But for patients who relapse or don’t respond to conventional treatments, the options are extremely limited, and less than half survive. For those patients, CAR-T cell therapy is a lifeline.
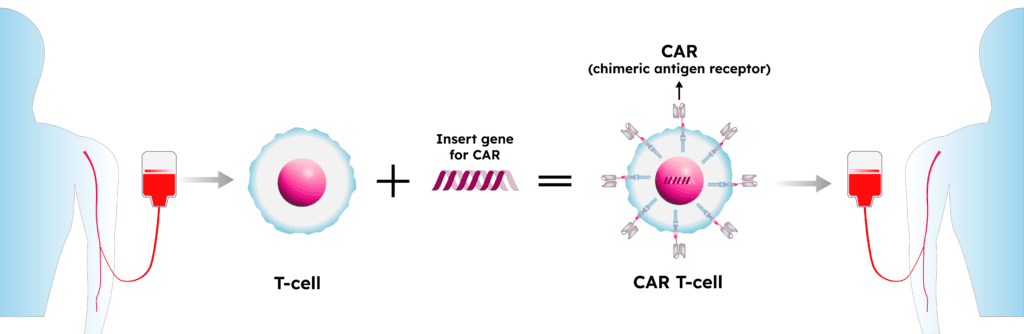
CAR-T cell therapy for ALL involves collecting a patient’s T cells—a type of immune cell—and genetically modifying them to express a protein that directs the T cells to kill leukemia cells that have a specific antigen on the surface. The modified T cells are infused back into a patient’s blood, where they target and destroy the leukemia cells. Data submitted to the FDA during the treatment’s approval process showed that in the first global clinical trial of CAR-T cell therapy, 82% of patients who received the treatment achieved remission.
Though ALL is the only pediatric cancer that CAR-T cell therapy is approved to treat at this time, there is research underway on extending its use to other types of pediatric cancers.
Cellular therapy at Phoenix Children's
Phoenix Children’s Center for Cancer and Blood Disorders (CCBD) has offered CAR-T cell therapy as a treatment option since 2017, when it became one of the first pediatric oncology programs in the nation approved to administer the treatment to children with relapsed or treatment-resistant B-cell acute lymphoblastic leukemia. The addition of CAR-T cell therapy has helped CCBD cement its reputation as one of the nation’s top pediatric cancer centers.