In spring 2019, Joshua and Ashley heard of a medical breakthrough that offered enormous promise for their family’s future. At the time, their 2-year-old son, Christopher, was learning how to walk with the support of crutches.
Christopher was born with spinal muscular atrophy (SMA), a genetic disease that causes severe muscle weakness. This condition can affect a child’s ability to walk, eat, communicate and breathe. In the worst cases, it can lead to infant death or a life spent immobile. Historically, there were no approved treatments for SMA, which affects one in every 10,000 babies. Patients were given nutritional and respiratory support, but their motor function would never improve.
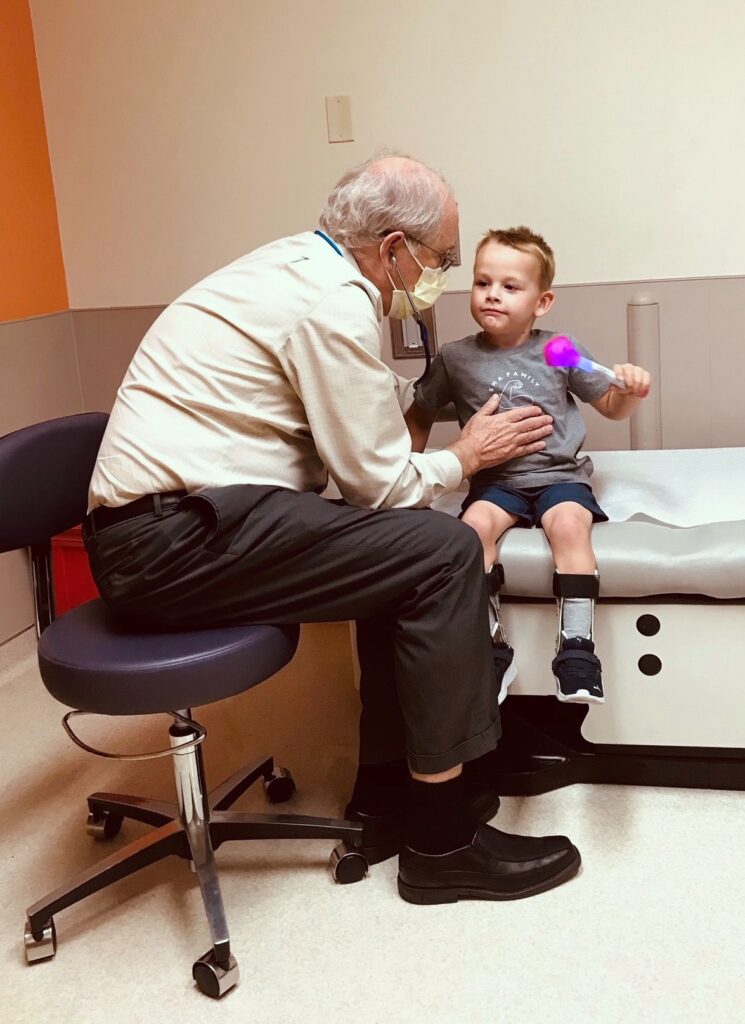
“It was very discouraging,” says Saunder Bernes, MD, a neurologist who has practiced at Phoenix Children’s for 37 years. “I believed there would be a treatment as science advanced—this was the hope I shared with families. But I didn’t expect a cure for presymptomatic babies. No one did.”
In May 2019, Zolgensma, a one-time gene-therapy treatment, was released. It is one of three drugs approved since 2016 that can open up once impossible realities for patients with SMA. Zolgensma works by adding a copy of the missing or malfunctioning gene in babies with SMA. If administered before symptoms start, there's a chance of normal motor outcome. “I tell families they should save for college. I would never have said this to parents who had a child with severe SMA 10 years ago,” says Bernes.
Because SMA is a genetic disease, Joshua and Ashley knew that if they had another baby, that child might have SMA, too. When they learned about Zolgensma, they felt comfortable with expanding their family, knowing that this revolutionary treatment was an option if Christopher’s younger sibling also was born with SMA.

In 2020, Christopher’s sister, Emily, was born. Within three days of her birth, a genetic test revealed that she too had SMA. “It should have been devastating news,” Ashley says. “But I felt hope in Zolgensma. I believed we could stop the disease in its tracks.”
When a newborn is diagnosed with SMA in Arizona north of Tucson, Phoenix Children’s is immediately notified to facilitate early care. Phoenix Children’s is one of the highest-volume treatment centers this side of the Mississippi, with 18 patients treated by Zolgensma as of September 2023, including Emily.
At 5 and a half weeks old, Emily went to Phoenix Children’s to receive her one and only dose of Zolgensma. “That day I remember feeling anxious and exhilarated,” says Ashley. “I didn't want to wait a year to see if she would take those first steps that Christopher missed out on, which is silly because as a mom, you want to enjoy those first few months.”
Emily, now 3, is symptom-free—she dances, paints and rides her scooter around the neighborhood. This fall, she started her first soccer season clad in her hot pink cleats. “Most of the time, we don’t even think about the fact that she has SMA,” says Joshua.


The first treatment for SMA was approved five months before Christopher’s birth. However, at the time, newborns were not routinely screened for SMA in the U.S. Ashley and Joshua didn’t realize something was awry until Christopher was slow to start walking.
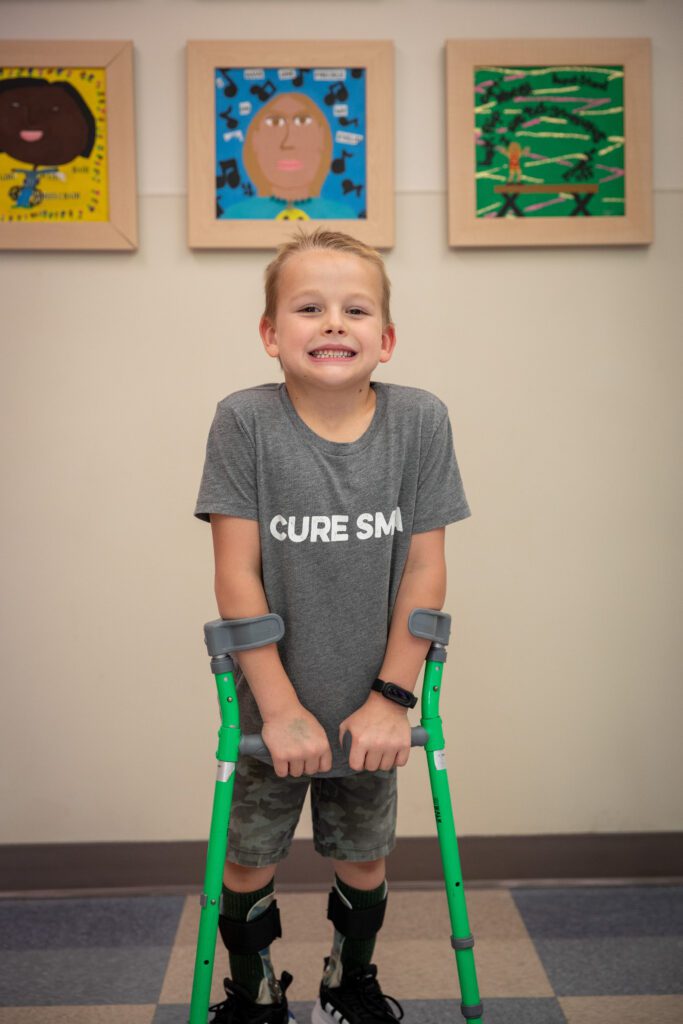
At 20 months of age, Christopher started treatment after displaying muscle weakness. “The bottom line is that presymptomatic treatment is better,” says Dr. Bernes. “Once motor or muscle function is lost, full restoration isn’t guaranteed.” Even though Christopher hasn’t met all his developmental milestones as he’s grown, his strength and mobility have progressed greatly thanks to clinical therapies.

“It’s been exciting to watch his development. I’ve known Christoper since he crawled and now, he can walk by himself, and his upper extremities are stronger or equal to most kids his age,” says Dr. Bernes. Joshua adds, “If Christopher hadn’t received treatment, now as a 6-year-old, he would likely be in a power wheelchair.”
Family members describe Christopher as a determined kid—he never complains about his condition, and he finds ways to participate in the activities he wants to do. Every month he comes to Phoenix Children’s for a procedure, and he approaches each poke with bravery and positivity.
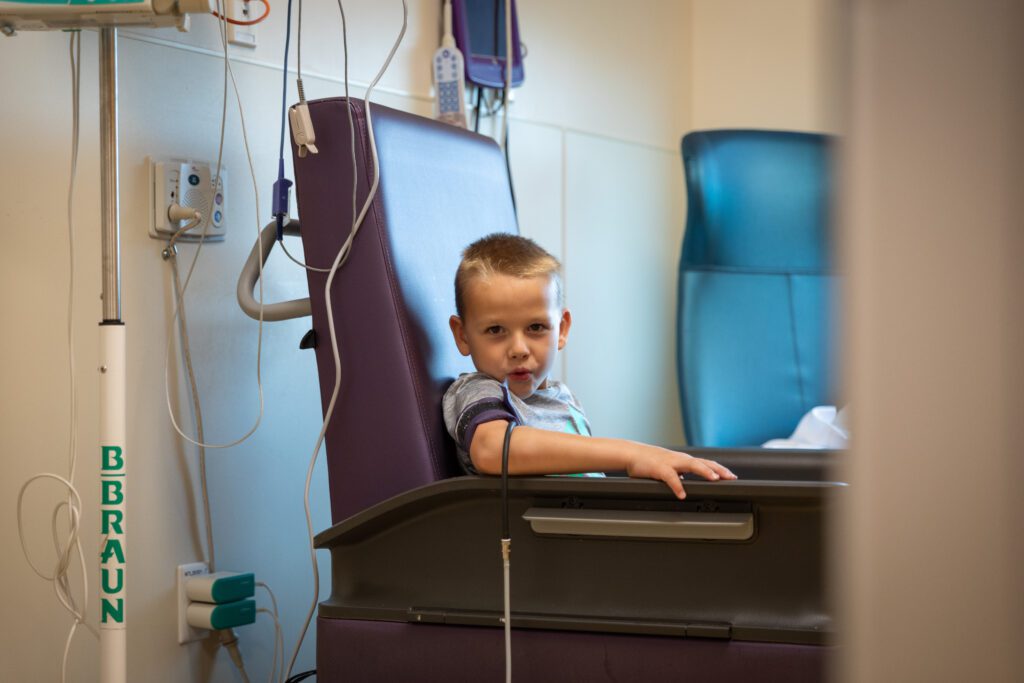
Research for spinal muscular atrophy is ongoing at Phoenix Children’s. There are multiple patients on clinical trial medications, including Christopher, who is taking one that helps his muscles grow. Another research program is working to decrease the risk of side effects associated with Zolgensma. The science surrounding SMA is advancing quickly, and according to Dr. Bernes, it’s likely that treatments available three to five years from now will be different from what exists today.
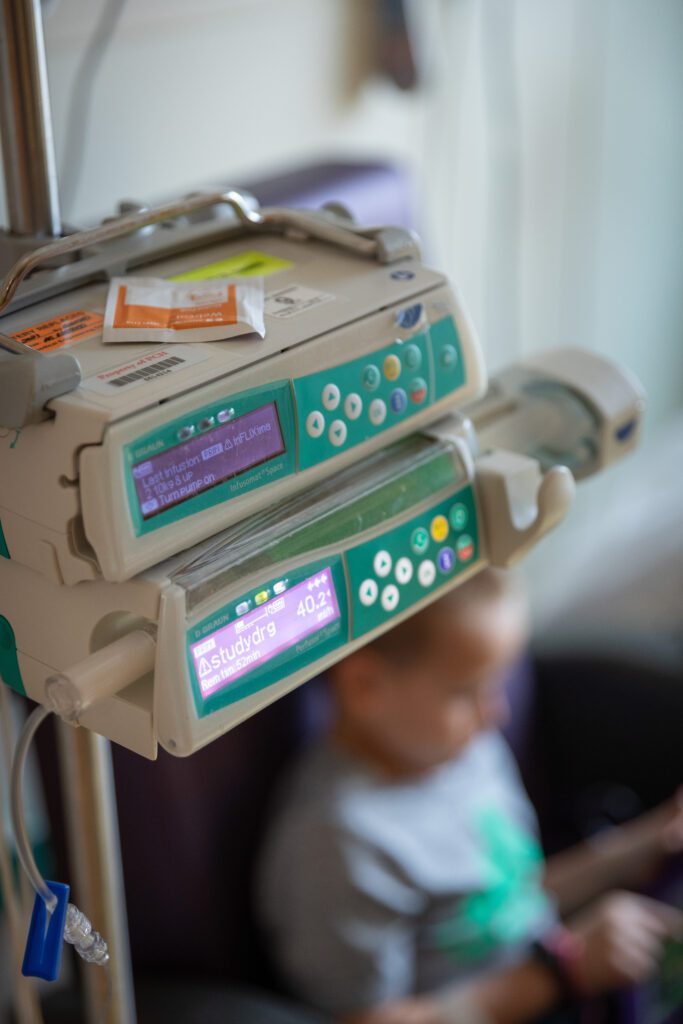
“The timing of everything is surreal. Having kids in this time when the medical field is exploding is a blessing, and having a hospital that keeps up with all the new treatments is even better,” says Ashley. Joshua adds, “I don’t know how we could have gotten through our situation without the help of Phoenix Children’s.”
At diagnosis, Ashley and Joshua didn’t know what the future would hold for their children. Today, when they see Christopher kick a soccer ball or Emily twirl in her favorite shoes, they are seeing the once impossible made possible.
Phoenix Children's has performed 18 Zolgensma treatments to date, effectively changing the course of life for children who would otherwise face an incurable, lifelong disability. Your philanthropy makes lifesaving and life-changing breakthroughs possible, creating the treatments and cures that will transform the lives of many families today and in the future.
Your gift helps the Barrow Neurological Institute at Phoenix Children's develop game-changing new treatments that will improve the quality of life of Arizona children living with complex neurological illnesses.